Glycan-targeting immunotherapy with AbLecs blocks sugar-driven immune suppression in cancer. See how AI can speed target discovery, design, and trials.
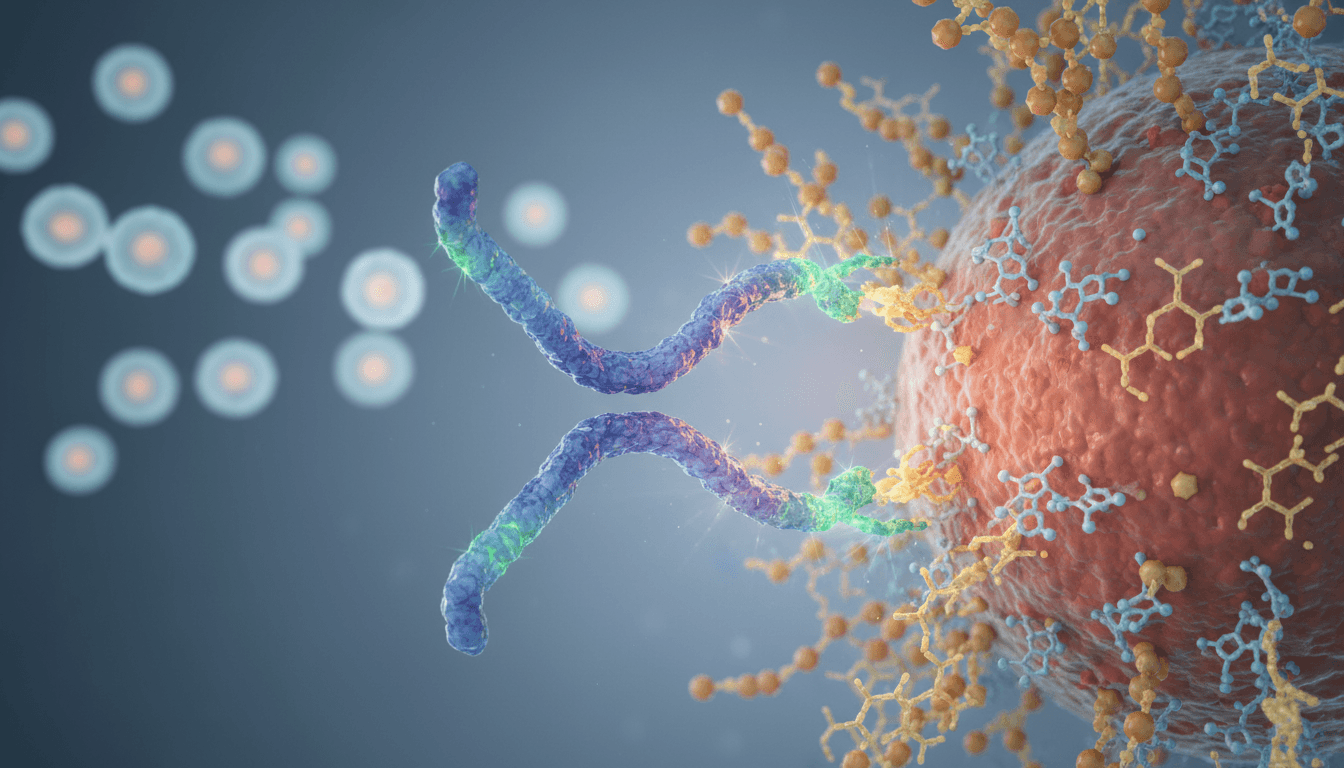
Glycan-Targeting Immunotherapy: How AbLecs Work
Most cancer immunotherapy strategies obsess over proteins. That’s understandable—proteins are familiar, measurable, and already drugged in countless ways. But tumors don’t just hide behind proteins. They also hide behind sugars.
A recent Nature Biotechnology research briefing (published 16 December 2025) spotlights an emerging modality called antibody–lectin chimeras (AbLecs). The headline isn’t subtle: these molecules bind and block tumor-associated glycans that drive immune suppression, and they’ve shown stronger antitumor immune activity than conventional antibodies—including therapies that are already approved.
For anyone following our “AI in Pharmaceuticals and Life Sciences” series, this is exactly the kind of development that should trigger a second thought: if the biology is more complex than proteins, then the discovery process has to get smarter too. Glycan biology is messy, context-dependent, and hard to measure at scale. That makes it a natural fit for AI-driven target discovery, molecule design, and clinical strategy.
Glycans are cancer’s “invisible ink” for immune suppression
Answer first: Tumors use altered glycosylation patterns to signal “don’t attack,” damping immune responses even when T cells are present.
Cancer cells frequently remodel the sugars decorating their surfaces and secreted proteins—glycans—creating patterns that immune cells interpret as self or safe. This isn’t just decoration. These glycans can engage immune receptors that deliver inhibitory signals, reducing macrophage killing, blunting antigen presentation, and weakening T-cell priming.
This matters because many patients now receive checkpoint inhibitors that primarily target protein-mediated brakes (like PD-1/PD-L1 pathways). Yet resistance and incomplete responses remain common across tumor types. One reason: a tumor can keep protein checkpoints quiet and still suppress immunity through glyco-immune checkpoints, such as sialic acid interactions with Siglec receptors.
What are “glyco-immune checkpoints” in plain terms?
Answer first: They’re inhibitory immune interactions driven by sugar structures rather than protein ligands.
If classic checkpoints are a handshake between proteins, glyco-immune checkpoints are closer to a barcode scan. Immune cells carry receptors (often lectin-like receptors) that “read” glycan patterns. Certain patterns—especially those enriched in sialylation—can communicate: slow down, don’t engulf, don’t inflame.
Two practical implications for drug developers:
- You can’t rely on gene expression alone to understand glycan-mediated suppression. Glycosylation is regulated post-translationally.
- Targeting one protein checkpoint may not be enough when a tumor’s glycan layer keeps immune cells restrained.
AbLecs: a new modality built to block glycan-driven immune evasion
Answer first: AbLecs combine antibody targeting with lectin-based glycan binding to physically block immunosuppressive glycans in the tumor microenvironment.
The AbLec concept is clever because it borrows the best parts of two biological tools:
- Antibodies provide specificity, pharmacokinetics, and established manufacturing know-how.
- Lectins naturally bind specific glycan motifs—exactly what you need if glycans are the functional “ligands” driving suppression.
By creating an antibody–lectin chimera, researchers aim to localize glycan blockade to relevant tumor contexts and interrupt inhibitory signaling that standard antibodies don’t touch.
The research briefing summarizes that AbLecs:
- Bind and block glycans linked to immune suppression in cancer
- Enhance antitumor immune responses in vitro and in vivo
- Outperform conventional antibody therapies, including approved cancer drugs (in the evaluated preclinical settings)
That last point is the one that should make R&D teams sit up. Outperforming approved antibodies in a controlled study doesn’t guarantee clinical success—but it’s a strong signal that the glycan layer has been under-targeted.
Why AbLecs are different from “yet another antibody”
Answer first: They expand the druggable surface area from proteins to the glycan code that controls immune behavior.
Traditional antibodies mostly care about protein epitopes. But tumors can evolve around protein targets (downregulation, mutation, antigen loss) and still maintain glycan-based suppression. AbLecs represent a strategic shift: treat the sugar coat as a functional immune interface.
That’s also why AbLecs may pair well with existing immunotherapies:
- Checkpoint inhibitors can remove protein brakes on T cells.
- AbLecs can remove glycan-mediated brakes on innate immune cells and antigen presentation.
Combination therapy is where immuno-oncology wins are often found—but only if you understand which suppressive axis dominates in a given tumor.
Where AI fits: glycans are high-dimensional, and humans can’t model them alone
Answer first: AI is well-suited to glycan-targeting immunotherapy because glycan structures, binding interactions, and patient variability create a data problem—more than a single-target problem.
Here’s my stance: glycan therapeutics will stall without serious computational support. Not because the biology isn’t real, but because the search space is huge and the measurements are noisy.
Glycans aren’t templated like proteins. They’re branched, heterogeneous, and influenced by cell state, metabolism, and microenvironment. Two tumors with similar mutations can display different glycosylation. And the same tumor can shift glycosylation under therapy pressure.
That complexity is exactly where AI earns its keep in pharma and life sciences:
1) AI for glycan target identification
Answer first: Machine learning can map glycan signatures to immune suppression phenotypes and prioritize targets that correlate with response and resistance.
Practical inputs that AI teams can fuse:
- Glycomics and glycoproteomics profiles (mass spec–derived)
- Single-cell RNA-seq (for enzymes controlling glycosylation and immune cell states)
- Spatial biology data (where glycan patterns sit relative to immune infiltrates)
- Histopathology + immunohistochemistry signals
- Real-world outcomes from immunotherapy-treated cohorts
The output isn’t just “this glycan exists.” It’s this glycan pattern predicts immune exclusion, or this Siglec axis correlates with PD-1 non-response. That’s a target strategy, not trivia.
2) AI for AbLec design and optimization
Answer first: AI can accelerate binder design by predicting lectin–glycan affinity/specificity and optimizing developability constraints.
AbLecs introduce design trade-offs: binding strength vs. specificity, tissue penetration vs. half-life, and potency vs. safety. AI approaches that matter here:
- Structure-based modeling to predict glycan-binding pockets and off-target glycan interactions
- Multi-objective optimization to balance potency, manufacturability, stability, and immunogenicity risk
- In silico developability screening (aggregation propensity, charge variants, viscosity risks)
This is where AI stops being a buzzword and becomes an R&D multiplier: fewer dead-end constructs, faster iteration cycles, and better preclinical candidate selection.
3) AI for clinical trial design in immuno-oncology
Answer first: AI can help match glycan-targeting therapies to the patients most likely to benefit and reduce false-negative trials.
Glycan biology varies across tumor types and patients. That’s a recipe for noisy trials unless you stratify well.
AI-enabled trial strategy can support:
- Biomarker-driven enrollment based on glycan signatures or receptor expression patterns (e.g., Siglec axis activity)
- Adaptive designs that adjust cohorts when early biomarker-response relationships appear
- Combination selection by predicting which immune suppressive pathways dominate (protein checkpoint vs. glycan checkpoint vs. myeloid suppression)
If you’re trying to generate leads in pharma and biotech, this is a strong conversation starter: How are you deciding which patients should receive a glycan-targeting modality—and how quickly can you prove it?
What drug developers should watch next (and what can go wrong)
Answer first: The promise is real, but glycan targeting raises predictable issues: specificity, safety, assays, and manufacturing consistency.
AbLecs are exciting precisely because they go after a neglected immune control layer. But there are four practical challenges that will decide whether this becomes a therapeutic class or a niche experiment.
Specificity: glycans exist on healthy tissue too
Answer first: The key risk is unintended binding to normal glycan patterns, especially in tissues with similar glycosylation motifs.
Unlike a mutated neoantigen, many glycan motifs are shared. The differentiator is often density, presentation, and microenvironment context—harder to capture than a single antigen expression value.
What helps:
- Careful glycan selectivity profiling across tissue panels
- Systems toxicology modeling and immune monitoring
- Patient selection where tumor-associated glycan signatures are clearly enriched
Safety: immune activation can spill over
Answer first: Enhancing immunity is the goal, but overstimulating innate responses can create inflammatory toxicity.
If AbLecs amplify macrophage activity and antigen presentation, you want that focused in the tumor. Monitoring cytokine patterns and myeloid activation markers will likely be central to early clinical development.
Assays: you can’t optimize what you can’t measure
Answer first: Robust glycan assays and functional immune readouts are make-or-break for development.
Teams will need fit-for-purpose assays to track:
- Target glycan occupancy / blockade
- Downstream receptor signaling changes (e.g., inhibitory lectin receptor pathways)
- Immune cell functional shifts in the tumor microenvironment
AI can help here too—especially by learning which assay combinations best predict in vivo response.
Manufacturing: heterogeneity isn’t only biological
Answer first: Complex biologics require tight control; chimera formats can introduce stability and consistency challenges.
Biologics manufacturing already wrestles with glycosylation consistency. Adding lectin components and specialized binding domains can increase sensitivity to process conditions.
If you work in pharma manufacturing or quality, you’ll recognize the opportunity: AI-driven process monitoring and quality control can reduce batch variability and speed deviation detection—another direct tie to our series theme.
Practical next steps for pharma and biotech teams evaluating glycan targeting
Answer first: Start by treating glycan immunotherapy like a platform decision: define the biology, measure it well, then design molecules and trials around the measurable signal.
If you’re exploring AbLecs or related glycan-targeting strategies, here’s a workable sequence that I’ve seen help teams avoid months of drifting:
- Pick a clear immune mechanism (e.g., myeloid suppression via inhibitory lectin receptors) and define the intended functional readout.
- Build a glycan measurement strategy early (glycomics + tissue validation + functional assays), not as a late add-on.
- Use AI to connect glycan patterns to outcomes, especially resistance to existing checkpoint inhibitors.
- Design for combinations from day one, because glycan checkpoint blockade may shine most when paired with protein checkpoint inhibitors or macrophage-directed therapies.
- Plan biomarker-driven trials to avoid averaging responders and non-responders into a disappointing “no signal.”
Snippet-worthy truth: If you can’t measure the glycan signal in patients, you don’t have a drug strategy—you have a hypothesis.
Where this fits in “AI in Pharmaceuticals and Life Sciences”
Glycan-targeting immunotherapy is a perfect stress test for AI in drug discovery and development. The biology is real, the datasets are messy, and the clinical stakes are high. That’s exactly where modern AI methods—applied carefully—can compress timelines and improve decision quality.
AbLecs also represent a broader trend we keep returning to in this series: new therapeutic modalities create new data and design problems. Companies that treat those problems as “just biology” tend to move slowly. Teams that pair modality innovation with strong AI and analytics capability tend to learn faster—especially in oncology, where time and signal-to-noise decide everything.
The next 12–24 months will tell us whether AbLecs become a repeatable platform across tumor types or remain confined to a narrow set of indications. The forward-looking question is simple: when glycan checkpoints become actionable, will your organization have the data, models, and trial strategy ready to move first?