AI smart bandages can monitor wounds every two hours and deliver electrical or drug therapy automatically. See what’s real, what’s next, and why it matters.
AI Smart Bandages That Treat Wounds in Real Time
A basic bandage does one job: cover the wound and hope the body does the rest. That “set it and forget it” approach is exactly why chronic wounds linger, infections sneak in, and clinicians end up making decisions with limited data between dressing changes.
A new proof-of-concept device called a-Heal points to a different future: an AI-guided smart bandage that monitors a wound, decides what it needs next, and delivers a targeted treatment automatically. The early results in a pig model were meaningful—50% new skin coverage vs. 20% in controls, plus a 61% reduction in an inflammation-related gene (interleukin 1 beta)—even though the study was small and didn’t run the device until full closure.
This post is part of our “Artificial Intelligence & Robotics: Transforming Industries Worldwide” series, and I’m using “robotics” intentionally here. A smart bandage like this isn’t a humanoid robot, but it is a robotic system in the way that matters: sensors + decision-making + actuation in a closed loop. That’s the pattern showing up across healthcare right now—automation that doesn’t replace clinicians, but tightens the feedback loop so care becomes faster, more consistent, and more personalized.
Why one-size-fits-all wound care fails
Wound healing isn’t linear, and it isn’t identical across patients. Standard dressings are built for coverage and moisture control, not for responding to what a wound is doing hour-by-hour.
Here’s where conventional wound care commonly breaks down:
- Sparse data: Clinicians see a wound during visits or dressing changes, then make decisions based on a snapshot.
- Delayed interventions: Inflammation or infection can ramp up between check-ins.
- Trial-and-error treatment: Many protocols rely on broad rules rather than patient-specific response.
- High burden settings: Think home care, rural care, long-term care facilities, and post-surgical recovery during the holidays when staffing is strained.
This matters in December 2025 for a practical reason: healthcare systems are still pushing hard on shorter lengths of stay and more care at home. The more we shift recovery out of hospitals, the more we need tools that keep quality high without requiring constant clinician presence.
What a-Heal is (and why it counts as AI + robotics)
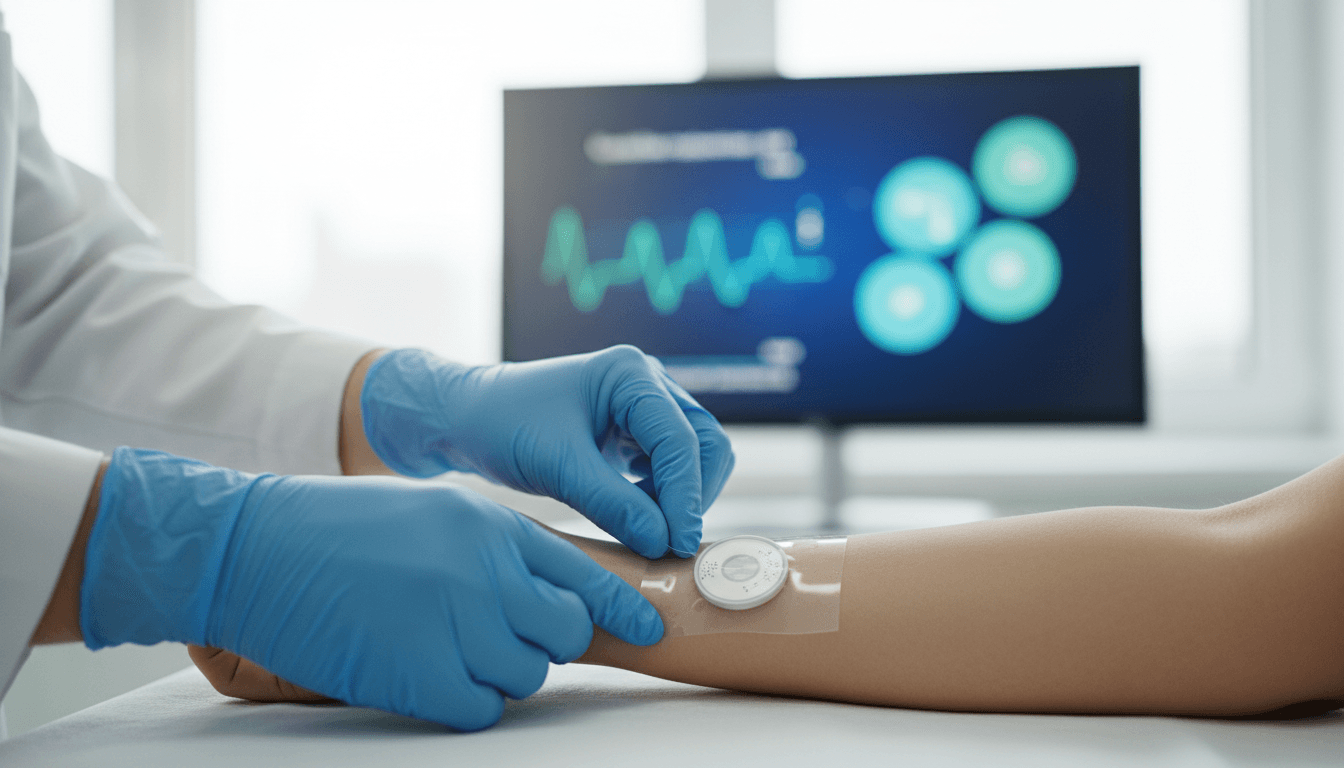
a-Heal is a closed-loop smart bandage that senses, decides, and acts. It was designed to fit inside a commercial colostomy bandage and includes:
- A camera module that images the wound every two hours
- A wireless link to a machine-learning system that evaluates healing stage
- Bioelectronic actuators that deliver one of two therapies based on the AI’s recommendation
The research team was led by Marco Rolandi at UC Santa Cruz, initially motivated by a DARPA goal: reduce battlefield wound healing time by 50%. Even if battlefield care isn’t your world, the same engineering goal maps neatly to civilian problems: pressure ulcers, diabetic foot ulcers, surgical wounds, and wounds complicated by infection.
The core idea: feedback control for biology
If you’ve worked with industrial automation, the pattern will feel familiar.
A smart bandage is essentially a feedback control system: measure the wound, classify the state, apply an intervention, then measure again.
That loop is the big shift. Instead of “pick a dressing and wait,” you get continuous adaptation—the same logic behind robotics in manufacturing lines or AI-controlled logistics systems, now applied to tissue repair.
How the AI makes treatment decisions
a-Heal uses images to estimate what healing stage the wound is in, then adjusts therapy accordingly. Every two hours, the device captures 11 images at different focal depths, feeding those into a machine-learning module the team calls ML Physician.
The system uses a leader–follower approach:
- Deep Mapper (the “leader”) predicts what the wound should look like at the next imaging timepoint.
- Reinforcement learning controllers (the “followers”) adjust treatment delivery to steer the wound toward that predicted trajectory.
This is a subtle but important design choice. The algorithm isn’t only classifying “good” vs. “bad.” It’s modeling a direction and then trying to keep healing on track.
Two interventions, one chosen at a time
The system selects one of two interventions (not both simultaneously):
- Electrical stimulation to reduce inflammation
- Drug delivery of fluoxetine (a drug associated with promoting tissue growth in the animal context used here)
The switching logic is clear and extractable: electrical stimulation is applied initially, and the system switches to drug delivery when the probability the wound is still in the inflammatory stage drops to 40%.
A practical takeaway: the value isn’t “AI picked a drug.” The value is consistent timing and dosage control in response to the wound’s state. Clinicians already know timing matters; machines just execute that timing with fewer gaps.
Dose control via iontophoresis
Delivery happens through iontophoresis, using current to drive molecules into tissue.
The actuator design is a cylindrical silicone polymer body with eight reservoirs arranged in a circle:
- Four reservoirs for electrical stimulation
- Four reservoirs for drug delivery
A hydrogel connects electrodes to the wound, and the wound bed becomes part of the circuit. By measuring current, the system estimates how many therapeutic molecules are delivered—an approach that supports precise dosing, not guesswork.
What the early results show (and what they don’t)
The early signal is promising, but it’s not a clinical validation yet. In the pig model:
- 50% of the treated wound area was covered by new skin cells vs. 20% for the control
- Interleukin 1 beta (an inflammation-associated gene) was reduced by 61%
- The device was used for 7 days within a 22-day experiment
- Wounds were not fully closed by day 22
External experts reacted with cautious optimism. One called the effect “modest” and wanted larger sample sizes and full-time use through closure to measure time-to-closure differences.
That critique is fair, and it’s also the normal path for medical devices: first, show feasibility and directionality; next, simplify hardware, scale studies, and prove outcomes that matter to payers and clinicians.
The real innovation is the combination
Electrical stimulation dressings exist. What’s new here is the photographic monitoring + machine learning + closed-loop actuation in one wearable system. That combination is exactly where healthcare robotics is heading: not one flashy component, but integrated systems that can run for days with minimal supervision.
Where AI smart bandages could land first: real-world use cases
If you’re evaluating AI in healthcare, the best starting point is high-cost, high-variance care. Wound care qualifies.
Here are the most plausible early applications, based on operational reality (not sci-fi):
1) Post-surgical monitoring at home
Hospitals want fewer readmissions; patients want fewer return visits. A smart bandage that flags “off-trajectory” healing could support:
- Earlier intervention for infection risk
- Fewer unnecessary clinic visits
- More objective handoffs between surgical teams and home health
2) Chronic wounds in long-term care
Pressure injuries and chronic ulcers are hard because progress is slow and staffing is variable. Closed-loop therapy could help standardize the basics:
- Consistent anti-inflammatory stimulation early
- Automated transition to pro-growth support
- Documentation that’s more robust than handwritten notes
3) Military and disaster medicine
The original DARPA motivation still makes sense. In austere settings, a system that can triage and treat with limited clinician time is a force multiplier—especially if it’s rugged, low-maintenance, and fast to deploy.
What needs to be true before this is mainstream
For AI-guided wound care to scale, it needs clinical trust, operational fit, and fairness. I’d look at five requirements.
1) Training data that reflects real skin tones and wound types
The team noted darker skin tone imaging should be “okay” if training data exists, but it’s too early to claim performance. This isn’t a box-checking issue. If the model misreads inflammation or tissue growth on certain skin tones, the closed loop could deliver the wrong therapy at the wrong time.
2) Outcomes that matter: time-to-closure and complications
Clinicians and payers will care about:
- Days to closure
- Infection rates
- Debridement and re-hospitalization rates
- Pain scores and scarring quality
“More re-epithelialization by day X” is a good start. It’s not the finish line.
3) Human-in-the-loop controls
Automation in healthcare works when it’s bounded. A strong commercial version would include:
- Clinician-configurable thresholds
- Override controls
- Treatment logs that are easy to audit
- Alerts when imaging quality degrades or the system is uncertain
4) Reliability, comfort, and manufacturability
The current prototype reportedly took about a month to build, and the team plans a flexible version. That’s not a minor detail. In the field, adoption will depend on:
- Wearability (movement, sweat, showering)
- Battery life and charging workflow
- Simple application by nurses and caregivers
- Cost per patient episode
5) Security and privacy by design
A camera that images wounds every two hours creates sensitive data. Any real deployment needs clear answers on:
- On-device vs. cloud processing
- Data retention and access controls
- Patient consent and caregiver training
What healthcare leaders can do now (even before smart bandages ship)
You don’t need this exact device to start benefiting from the same operating model. The big lesson is closed-loop care: measure frequently, decide consistently, act quickly.
If you run a clinic, hospital service line, or digital health program, here are practical next steps:
- Standardize wound imaging workflows (lighting, distance, angle) so future AI tools can plug into cleaner data.
- Define “trajectory metrics” your team agrees on (expected improvement per week, escalation thresholds).
- Pilot decision support before automation: start with AI that recommends interventions, then add actuation later.
- Map reimbursement and documentation needs early. Many AI pilots fail because they can’t fit into billing and compliance workflows.
- Design for at-home care: assume the user is a caregiver on a tight schedule, not a wound specialist.
The fastest path to ROI in healthcare AI is shrinking the time between “something changed” and “someone acted.”
What this means for AI and robotics across industries
This smart bandage story fits the broader theme of our series: AI and robotics are moving from standalone tools to integrated systems that run continuous loops—sense, decide, act. We’re seeing the same pattern in warehouse picking robots, predictive maintenance in factories, and now in clinical recovery.
Healthcare is slower for good reasons (safety, validation, regulation). But when closed-loop systems work, they create a new baseline for quality: fewer missed signals, more consistent execution, and care plans that adapt to the individual.
The next question isn’t whether bandages will “think.” It’s who will build the care workflows that let smart devices help without turning clinicians into system babysitters.